
Catch Validation Failures Before They Delay Your FDA Submission
Advanced simulation for medical device teams who need fewer iterations, stronger documentation, and faster regulatory clearance.
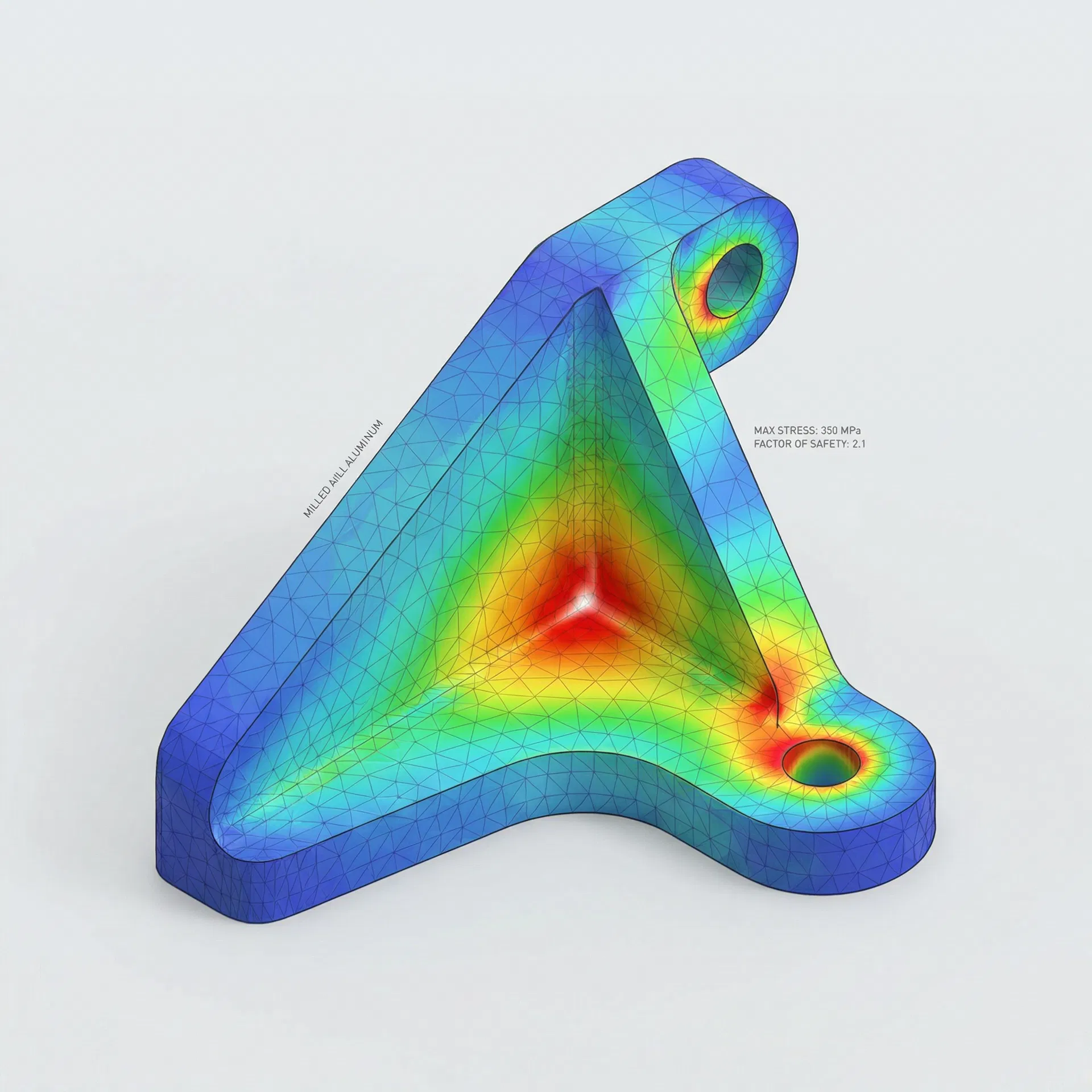
The True Cost of Late-Stage Validation Failures
When design flaws surface during design verification testing instead of during CAD, the impact cascades across your entire timeline and budget.
$50k–$200k Per Iteration
Injection molds, extrusion tooling, biocompatible material costs, and test fixture fabrication add up fast—especially when you discover the failure after tooling is cut.
8–12 Weeks Lost
Each prototype iteration pushes design freeze back by 2-3 months. Supplier lead times, lab scheduling, and rework cycles compound into submission delays you can't recover.
Competitive Risk
While you're rebuilding prototypes, competitors are filing 510(k)s. In fast-moving device categories, being second to market can mean losing the entire opportunity.
Every failed DVT build doesn't just cost money—it burns runway, delays revenue, and gives competitors a 6-month head start.
Physical Prototyping Is Expensive, Slow, and Risky
Medical device prototyping carries hidden costs that compound with every iteration. Here's what most R&D teams don't budget for:
Tooling & Molds
$30k–$150k for injection molds, extrusion dies, or custom fixtures—often non-reusable if design changes.
Biocompatible Materials
Medical-grade PEEK, titanium, or silicone at 10–50× the cost of standard materials, with minimum order quantities.
Lab Time & Testing
$15k–$40k per test cycle for fatigue, sterilization validation, and mechanical characterization.
Supplier Lead Times
6–10 weeks for custom components, with no guarantee the design will pass testing once delivered.
Engineering Bandwidth
Your team spends weeks managing vendors, scheduling tests, and troubleshooting failures instead of designing.
Rework Cycles
Each design change triggers a new round of tooling, materials, and testing—multiplying costs exponentially.
Most medical device companies budget for 2–3 prototype iterations. Reality: Without simulation, you'll need 5–7 iterations to reach DVT-passing confidence.
FDA Timelines Are Unforgiving. Validation Delays Compound Fast.
Regulatory submission windows are fixed. Prototype failures push design freeze back, compressing the time you have to prepare your technical file and complete design verification testing.
The Compliance Pressure
- 510(k) submissions require complete design verification evidence—no shortcuts.
- PMA pathways demand even more rigorous validation data and clinical evidence.
- Design history files must document every design decision—including why you chose specific materials and geometries.
- Late-stage design changes trigger re-validation of the entire test protocol.
The Timeline Reality
- Investors expect 12–18 month development cycles from concept to submission.
- Each prototype failure adds 8–12 weeks to your timeline—compounding with every iteration.
- Clinical partners and distribution agreements are often contingent on hitting submission deadlines.
- Missing your window means competitors file first—and in many device categories, first-to-market captures 60%+ of the opportunity.
You can't negotiate with the FDA on timelines. But you can control how fast you reach design freeze.
How Early Simulation Eliminates Late-Stage Risk
Digital validation moves failure discovery from the lab back into CAD—where fixes cost hours instead of months and thousands instead of hundreds of thousands.
Validate Before You Build
Test fatigue at 10 million cycles, sterilization thermal stress, and insertion forces digitally—before ordering a single prototype.
Run Parallel Scenarios
Evaluate multiple material choices, wall thicknesses, and geometry variations simultaneously—something impossible with physical testing.
Optimize for Manufacturing
Identify stress concentrations, optimize rib placement, and validate draft angles before cutting tooling.
Build FDA-Compliant Documentation
Simulation results integrate directly into design history files, providing traceable evidence of design decisions.
The Simulation Advantage
"We went from 4 failed prototypes to passing DVT on our first build. Simulation saved us 6 months and $180k."
— R&D Director, Class II Orthopedic Device Company
Real Results: From 7 Prototypes to 1
How a venture-backed Class III cardiovascular device company used simulation to eliminate $300k in prototyping costs and accelerate their PMA submission by 8 months.
The Challenge
A cardiovascular device startup was developing a polymer catheter with a nitinol core. Their initial prototypes kept failing fatigue testing at 500k cycles—far short of the 10 million cycle requirement. Each iteration required new extrusion tooling ($45k), biocompatible polymer batches ($18k), and 10 weeks of lead time. After three failed builds, they were 7 months behind schedule and burning through their Series A runway.
The Simulation Approach
SWYFT Solutions implemented SOLIDWORKS Simulation to model the catheter's hyperelastic polymer behavior and nitinol core interaction under cyclic loading. We ran 15 design variations in parallel, testing different wall thicknesses, core diameters, and polymer durometers digitally.
- Identified stress concentration at the polymer-nitinol interface causing premature failure
- Optimized wall thickness taper to distribute stress more evenly
- Validated sterilization thermal stress wouldn't degrade polymer properties
- Predicted fatigue life at 12+ million cycles before building a single prototype
The Outcome
"Simulation didn't just save us money—it saved our company. We hit our PMA submission deadline and closed our Series B on the strength of that timeline."
— VP of Engineering, Cardiovascular Device Startup (name withheld per NDA)
The Right Simulation Power for Your Device Complexity
From Class I device housings to Class III implantables with hyperelastic materials and tissue contact—we match the simulation tool to your validation requirements.
SOLIDWORKS Premium
Integrated FEA
Best for:
- Class I & II device housings
- Handheld instrument stress checks
- Rapid drop-test validation
Validate inside CAD without leaving SOLIDWORKS.
Catch basic structural issues before prototyping.
Build confidence for first design verification builds.
SOLIDWORKS Simulation
Advanced Analysis
Best for:
- Class II & III implantable devices
- 10M+ cycle fatigue validation
- Sterilization thermal stress analysis
- Multi-component assembly contact
Test sterilization, fatigue, and insertion in parallel.
Reach design freeze faster with fewer prototypes.
Build DVT-ready designs on first physical iteration.
SIMULIA
High-Fidelity / Abaqus-Level Power
Built for high-risk, high-complexity devices:
- Hyperelastic silicone & polymer catheters
- Large-deformation insertion mechanics
- Tissue-device contact & friction modeling
- Composite stent & scaffold structures
- Coupled thermal-mechanical-fluid systems
When your device interacts with human tissue, failure prediction must be surgical-grade precise.
SIMULIA (Abaqus) delivers the solver fidelity that venture-backed medtech companies trust for Class III submissions.
Ready to Eliminate Preventable Prototype Failures?
Bring us one critical component from your device. We'll walk through your design verification requirements, show you where simulation can replace physical testing, and help you build an FDA-compliant validation strategy that accelerates your timeline.
SWYFT Solutions | Authorized SOLIDWORKS Reseller
616-631-3044 | [email protected]